Since their first appearance in the 1960s, liquid motion lamps have been a familiar fixture in college dorms and teenagers' bedrooms all over the world. In the United States and many other countries, the novelty devices have become completely entrenched in popular culture. Even after all these years, people are still buying motion lamps, and the major manufacturers now offer hundreds of variations on the basic design!
In this article, we'll look at these popular devices to find out exactly what happens inside to produce such a mesmerizing display. We'll also explore some of the history behind liquid motion lamps, and even get you started on making your own basic lamp. The next time you see a motion lamp, you'll definitely be transfixed, because you'll know all about the amazing processes at work.
Advertisement
Inside the Lamp
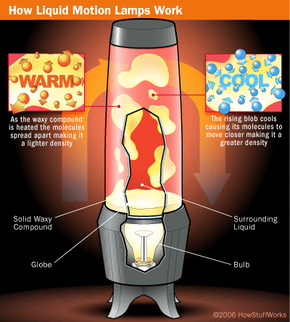
Liquid motion lamps are actually fairly simple devices. They are based on very basic scientific principles and consist of only a few simple components. They must have:
- A compound that makes up the floating "blobs"
- A compound that the blobs float in
- A lamp that illuminates the display and provides the heat necessary to move the blobs
To create the floating blobs, the two compounds in a motion lamp must be immiscible, or mutually insoluble. All this means is that liquid A doesn't dissolve in liquid B -- the two don't mix, so you see two separate liquids, one floating on top of or within the other.
The classic example of immiscible compounds is oil and water. If you fill a jar with common mineral oil and water, you'll get a water layer with a layer of oil floating above it. This combination of water and oil in a jar has a similar look to a commercial motion lamp with its light turned off; in a cold lamp you see two separate layers.
The coolest thing about motion lamps, of course, is that they produce distinct amorphous blobs that rise and fall in the lamp's "globe" on their own. To produce this effect, you need to pick your two insoluble compounds very carefully. In our oil and water jar, the water ends up on the bottom because it has a much higher density than oil. Simply put, a liquid with a higher density pushes a lower-density liquid upward (for more on this, check out How Helium Balloons Work).
To get blobs that will float around, you need two substances that are very similar in density, so that the blobs can easily switch between rising and sinking. Then you need to be able to change the density of one of the compounds so that sometimes it is lighter than the other compound (and so floats to the top) and sometimes it is heavier (so that it sinks to the bottom). We'll look at how to do this in the next section.
Advertisement