Key Takeaways
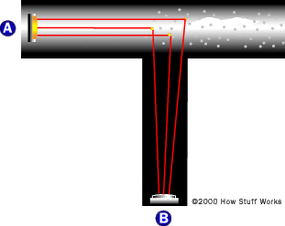
- Smoke detectors contain a light source and sensor positioned at 90-degree angles. When smoke enters, it scatters light onto the sensor, triggering the alarm.
- These devices are particularly effective at sensing smoky fires that smolder, like those that might occur in a mattress, due to their sensitivity to smoke particles scattering light.
- The mechanism contrasts with simpler beam-interrupting systems, offering more sensitivity and earlier detection by requiring less smoke to trigger the alarm.
Smoke detectors are one of those amazing inventions that, because of mass production, cost practically nothing. You can get a smoke detector for as little as $7. And while they cost very little, smoke detectors save thousands of lives each year. In fact,it is recommended that every home have one smoke detector per floor.
All smoke detectors consist of two basic parts: a sensor to sense the smoke and a very loud electronic horn to wake people up. Smoke detectors can run off of a 9-volt battery or 120-volt house current.
Advertisement
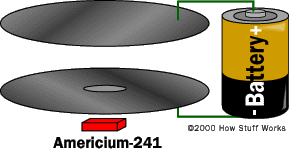
In this article, we will examine the two most common types of smoke detectors used today: photoelectric detectors and ionization detectors. And, we'll also take a look inside an ionization detector.
Let's start with photoelectric detectors.
Advertisement