A Bright Future
Electrochromic windows darken when voltage is added and are transparent when voltage is taken away. Like suspended particle devices, electrochromic windows can be adjusted to allow varying levels of visibility. They are not an all-or-nothing technology like liquid crystals.
Electrochromic windows center around special materials that have electrochromic properties. "Electrochromic" describes materials that can change color when energized by an electrical current. Essentially, electricity kicks off a chemical reaction in this sort of material. This reaction (like any chemical reaction) changes the properties of the material. In this case, the reaction changes the way the material reflects and absorbs light. In some electrochromic materials, the change is between different colors. In electrochromic windows, the material changes between colored (reflecting light of some color) and transparent (not reflecting any light).
Advertisement
At its most basic level, an electrochromic window needs this sort of electrochromic material and an electrode system to change its chemical state from colored to transparent and back again. There are several different ways to do this, employing different materials and electrode systems.
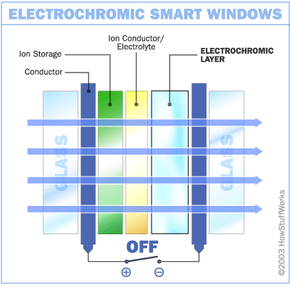
Like other smart windows, electrochromic windows are made by sandwiching certain materials between two panes of glass. Here are the materials inside one basic electrochromic window system and the order you will find them in:
- Glass or plastic panel
- Conducting oxide
- Electrochromic layer, such as tungsten oxide
- Ion conductor/electrolyte
- Ion storage
- A second layer of conducting oxide
- A second glass or plastic panel
In this design, the chemical reaction at work is an oxidation reaction -- a reaction in which molecules in a compound lose an electron. Ions in the sandwiched electrochromic layer are what allow it to change from opaque to transparent. It's these ions that allow it to absorb light. A power source is wired to the two conducting oxide layers, and a voltage drives the ions from the ion storage layer, through the ion conducting layer and into the electrochromic layer. This makes the glass opaque. By shutting off the voltage, the ions are driven out of the electrochromic layers and into the ion storage layer. When the ions leave the electrochromic layer, the window regains its transparency.
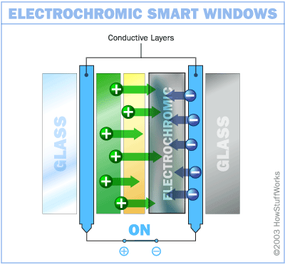
With an electrochromic smart window, it only requires electricity to make the initial change in opacity. Maintaining a particular shade does not require constant voltage. You merely need to apply enough voltage to make the change, and then enough to reverse the change -- making this pretty energy-efficient. In fact, according to Sage Electronics, you can run a house full of electrochromic windows for about the same amount of money that it takes to power a single 75-watt light bulb.

Although they can technically be classified as electrochromic materials, the new reflective hydrides that are being developed behave in a noticibly different way. Instead of absorbing light, they reflect it. Thin films made of nickel-magnesium alloy are able to switch back and forth from a transparent to a reflective state. The switch can be powered by low-voltage electricity (electrochromic technology) or by the injection of hydrogen and oxygen gases (gas-chromic technology). Furthermore, this material has the potential to be even more energy efficient than other electrochromic materials.
We're surrounded by windows every day, but we probably don't stop to think about them very often. With advances in smart window technologies, we will start to see windows in a whole new light.
For more information on smart windows and related topics, check out the links below.
Related Articles
More Great Links
- "Toolbelt Diva"
- Research Frontiers
- SwitchLite Privacy Glass™
- SageGlass Electrochromic Windows
- Windows & Daylighting: Chromogenic Materials