Let's say you take a glass of ice water or a bowl of hot soup and let them sit out on the kitchen table. You know what will happen: The bowl of soup will cool down to room temperature, and the glass of ice water will warm up to room temperature. This is a thermodynamic fact of life -- if you put any two objects with different temperatures together, then heat transfer will cause them to reach the same temperature. So a "room" and a "hot bowl of soup" reach the same temperature by the heat transfer process -- the room gets slightly warmer and the bowl of soup gets a lot colder.
If you want to keep a bowl of soup hot as long as possible -- that is, if you want to slow down the natural heat transfer process as much as you can -- you have to slow down the three processes that cause heat transfer. The processes are:
- Conduction - Let's start with a simple question: What is heat? Heat is atomic motion. An atom represents its "heat" by its speed. At the temperature absolute zero, there is no atomic motion. But as atoms get warmer they move. Heat is transferred when one atom runs into another. When this happens, it is a little bit like billiard balls colliding -- the second atom picks up some of the motion of the first atom. Heat is transferred by these collisions.
The best example of this phenomenon would be to take a metal bar and heat one end of it. The other end will get warm and then hot through conduction. When you put a metal pan on the stove, the inside of the pan gets hot through conduction of the heat through the metal in the bottom of the pan. Some materials (namely metals) are better heat conductors than others (for example, plastics).
- Radiation - Another side effect of atomic motion is vibration, and vibration leads to the unexpected phenomenon of infrared radiation. According to the Encyclopedia Britannica, "Infrared radiation is absorbed and emitted by the rotations and vibrations of chemically bonded atoms or groups of atoms and thus by many kinds of materials." Infrared radiation is a form of light.
Our eyes are unable to see infrared, but our skin can feel it. About half of all of the sun's energy that reaches us comes as invisible infrared radiation, with the rest of it visible to us as light. Infrared, like visible light, is reflected by mirrors and absorbed better by black objects. When infrared is absorbed, it results in atomic motion, and therefore, in a rise in temperature. Some common examples of infrared are the heat you feel radiating from an electric heater or a red-hot piece of metal, the heat you feel radiating from the bricks in a fireplace even if the fire has gone out and the heat you feel radiating from a concrete wall after the sun has gone down.
- Convection - Convection is a property of liquids and gases. It occurs because when a liquid or gas gets hot, it tends to rise above the rest of the body of liquid or gas. So, if you have a hot bowl of soup on the table, it heats a layer of air surrounding the bowl. That layer then rises because it is hotter than the surrounding air. Cold air fills in the space left by the rising hot air. This new cold air then heats up and rises, and the cycle repeats. It is possible to speed up convection -- that is why you blow on hot soup to cool it down. If it weren't for convection your soup would stay hot a lot longer, because it turns out that air is a pretty poor heat conductor.
You can see all three of these heat transfer processes occurring when you stand next to a bonfire:
You probably need to stand at least 20 feet away from a big bonfire like this one. What keeps you away is heat radiating from the fire through infrared radiation. The flames and smoke are carried upward by convection: Air around the fire heats up and rises. The ground 3 feet beneath the fire will get hot, heated by conduction. The top layer of soil is directly heated (by radiation), and then the heat is conducted through layers of dirt deep into the ground.
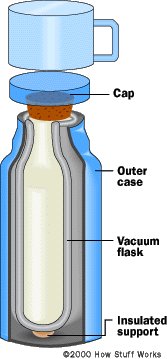
To build a good thermos, what you want to do is reduce these three heat transfer phenomena as much as possible.