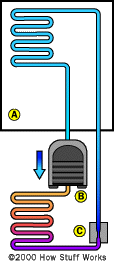
The next time you indulge in an ice cold drink on a hot day, you have your refrigerator (and onboard freezer) to thank for the refreshingly chilled beverage. It wasn't so long ago that you'd have to be very rich or well connected to score a chilled drink with a few ice cubes floating inside. Today, we take refrigeration for granted, but once upon a time, fortunes were made shipping large blocks of ice around the world in insulated holds to sell to the rich.
Before refrigeration, preserving food was a big job. You could salt foods, and in winter, you could bury food in a snow drift and hope the critters didn't find it. To stay stocked with the essentials, though, you had to work at it -- or be rolling in money. Refrigeration is one invention that changed the way we conduct our daily lives. We can preserve food more easily nowadays, so we have much less to worry about when it comes to food-borne illnesses. The food supply is more stable, too. That gallon of milk can last a couple of weeks in the fridge as opposed to a couple of hours on your countertop. That's huge. It means you don't need to keep a cow in your backyard if you want a regular supply of milk.
Advertisement
The fundamentals of refrigeration are also at work in another important household appliance: the air conditioner. It's estimated that around 5 percent of all the electrical energy used in the U.S. is expended to keep our homes cool. That's pretty amazing, especially when you consider the fact that the principle behind most refrigeration is simple. Here it is in one sentence: When a liquid evaporates, it absorbs heat in the process. If you want to get rid of heat, you need to coax a liquid to convert to its gaseous state [source: ACEEE].